5-Min Science: Demystifying the Insular Cortex
- BioSource Faculty
- Feb 8
- 17 min read
Updated: Feb 11
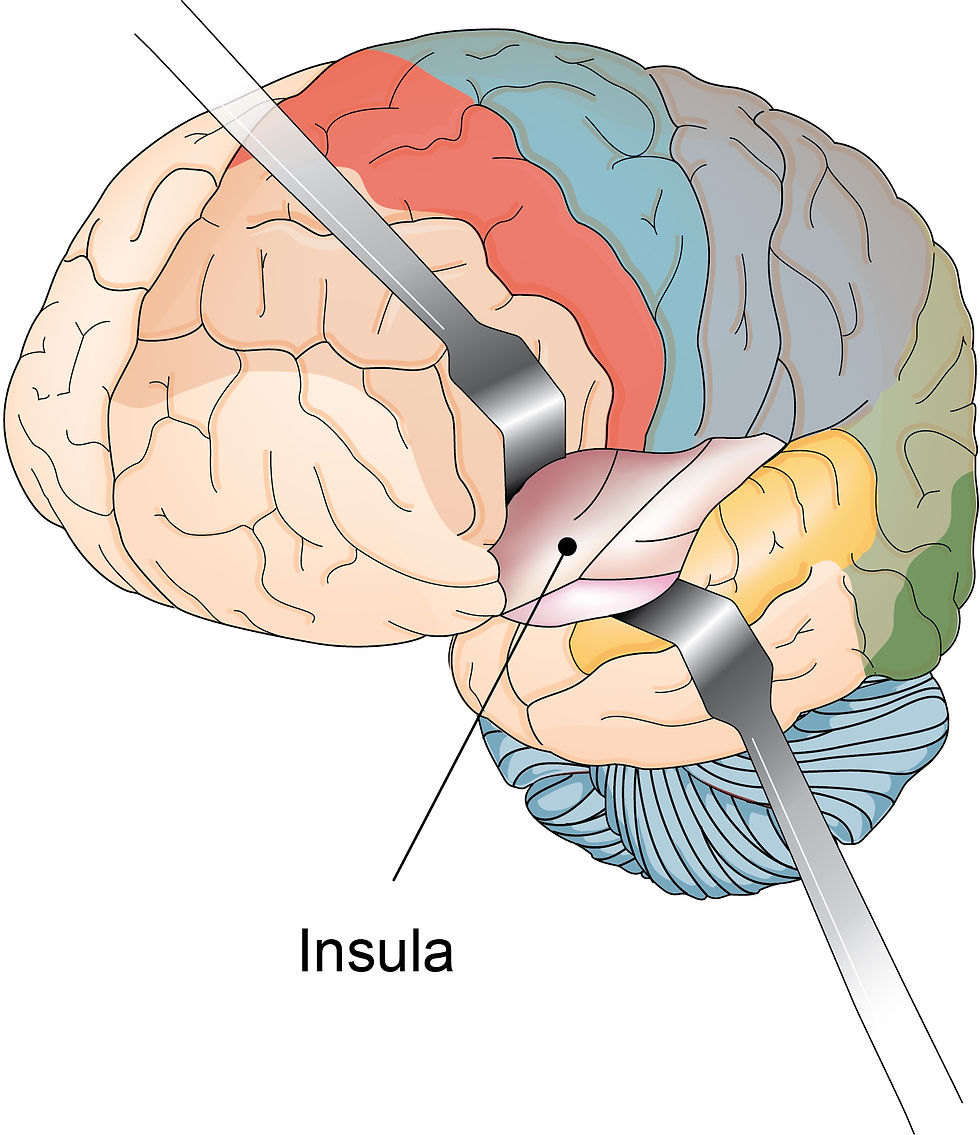
Introduction
The insular cortex is your brain's integration hub for bodily sensations, emotions, and thoughts.
Imagine you're anxious about giving a presentation. Your insula is registering your racing heart, the butterflies in your stomach (interoception), combining this with your emotional memories of past presentations and helping create your conscious experience of anxiety. This integration happens through three main sections.
The posterior insula (at the back) acts like a sensory reception desk, taking in raw data about your body's state - pain, temperature, hunger, and other internal sensations. This information travels forward to the middle insula, which begins processing these signals into more complex patterns.
The anterior insula (at the front) is where things get really interesting. It has two parts: The dorsal (upper) portion works with attention and cognitive control, while the ventral (lower) portion processes emotions. Together, they transform all this information into conscious experiences - helping you know how you feel at any given moment.
Why the Insular Cortex Is Poorly Understood
The insular cortex remains less understood than other cortical regions largely because its concealed location within the Sylvian fissure complicates imaging and accessibility (Gharizi et al., 2018; Nieuwenhuys, 2012). Its complex cytoarchitecture and multifaceted connectivity underpin a broad range of functions—from sensory processing and emotional regulation to interoception and pain perception—making it difficult to assign discrete roles (Benaroch, 2019; Schelley & Trimble, 2004). Furthermore, its unique evolutionary history and variability across species, combined with extensive links to frontal, temporal, parietal, occipital, limbic, and subcortical areas, add to the overall complexity (Cloutman et al., 2012; Gharizi et al., 2017, 2018).
The Insula’s Location and Organization
Situated deep within the lateral sulcus, the insula is traditionally divided into anterior and posterior regions. Recent studies, however, suggest a tripartite division into the dorsal anterior, ventral anterior, and posterior areas (Nomi et al., 2016, 2018). The dorsal anterior insula connects with frontal regions to support cognitive control and attention (Haruki & Ogawa, 2021), while the ventral anterior insula, linked to limbic structures, facilitates emotional processing (Nomi et al., 2018; Zhang et al., 2021). The posterior insula, which receives diverse sensory inputs such as pain and temperature, plays a central role in sensorimotor and interoceptive functions, with the middle insula serving as a transitional zone that refines these signals (Haruki & Ogawa, 2021; Nomi et al., 2018).

Network Connections
The insula functions as a pivotal node within several brain networks. As part of the salience network, the fronto-insular cortex mediates the switch between the default mode and central executive networks, thereby guiding attention and cognitive performance (Li et al., 2018; Uddin et al., 2014). Its posterior region contributes to the sensorimotor network by processing sensory information, while mid-insular areas integrate these signals with emotional and cognitive inputs to modulate pain (Bennaroch, 2019; Lu et al., 2016). Additionally, the dorsal anterior insula plays a central role in cognitive networks by engaging in emotional, memory, attention, and reasoning tasks. It is linked with the frontoparietal network to support cognitive control (Kurth et al., 2010; Menon et al., 2020; Sridharan, Levitin, & Menon, 2008). The salience and frontoparietal networks from Schimmelpfennig and colleagues (2023) are shown below. Creative Commons Attribution License (CC BY).

Attention
In attentional processes, the anterior insula is essential within the salience network, where it detects and prioritizes significant stimuli and mediates network switching for efficient resource allocation (Menon & Uddin, 2010; Nelson et al., 2010). Its functional connectivity with regions such as the right temporoparietal junction and dorsal prefrontal cortex reinforces its role in focus management, with disruptions in these networks leading to heightened distractibility (Nelson et al., 2010). Intracranial EEG studies further confirm the anterior insula’s involvement in voluntary, top‐down processing of task-relevant information, particularly under conditions of sleep deprivation (Centanni et al., 2021; Nelson et al., 2010). The temporal parietal junction, Simone Vossel, Joy J. Geng, Gereon R. Fink, CC BY 3.0, via Wikimedia Commons, is shown below.

Mindfulness
Mindfulness practices engage the insula by enhancing interoception and emotional regulation. Mindfulness-based interventions increase insular activity (Young et al., 2018; Zsadanyi, Kurth, & Luders, 2021), with the anterior insula being critical for re-representing interoceptive signals that underpin present-moment awareness and facilitating a detachment from self-referential processing (Wheeler, Arnkoff, & Glass, 2017). Moreover, structural changes such as increased cortical thickness in the posterior insula and altered connectivity with attentional and sensory networks further attest to its role in mindfulness (Wheeler, Armkoff, & Glass, 2017; Zsadanyi, Kurth, & Luders, 2021).
Empathy
Empathy and socially guided emotional behavior are rooted in the insula’s ability to integrate emotional, cognitive, and sensory inputs. The anterior insula, in particular, supports both cognitive and affective empathy by mediating shared neural representations between action systems and limbic structures (Singer, Critchley, & Preuschoff, 2009). Its connectivity with the anterior cingulate cortex and modulation by contextual factors like perceived fairness underscore its importance in adaptive social interactions and decision-making (Bernhardt & Singer, 2012).
Emotion
The insular cortex integrates interoceptive signals with higher cognitive processes to generate conscious emotional experiences. Working alongside the anterior cingulate cortex, the anterior insula is instrumental in recognizing and processing emotions such as anger and sadness (Gu et al., 2013; Jumah, 2018). Lateralized functions are also evident; for instance, the right insula is crucial for sympathetic arousal and cognitive-affective processing, indicating asymmetrical roles in emotional evaluation (Bennaroch, 2019; Zhang et al., 2019). This integration reinforces its centrality within the brain’s emotional and cognitive networks (Cloutman et al., 2012).
Decision-Making
Decision-making relies on the insula’s capacity to integrate sensory, emotional, and cognitive information across various stages—from attention refocusing to outcome evaluation. The anterior insula’s integration of cognitive and emotional signals is reflected in its high-frequency activity during decision tasks, whereas the posterior insula contributes somatosensory input for initial evaluative processes (Llorens et al., 2022). Damage to the insula disrupts risk assessment and decision patterns, emphasizing its role in processing uncertainty and modulating subjective states through error-based learning (Castelhano et al., 2018; Naqvi & Bechara, 2009).
Interoception
Central to interoception, the insular cortex integrates internal bodily signals to maintain physiological homeostasis, emotional regulation, and cognitive function (Wang et al., 2018, 2019). The anterior insula exhibits focused connectivity with somatosensory areas during tasks requiring interoceptive attention, in contrast to its reduced connectivity with visual regions, highlighting its specialized role. Moreover, the insula fuses interoceptive and exteroceptive inputs—such as olfactory signals processed by the central dorsal insula—to create a coherent perception of bodily states, which is essential for predicting future physiological conditions through interactions with the hypothalamus and amygdala (Benarroch, 2019; Evrard, 2019).
Emotional and Physical Pain
The insula integrates sensory-discriminative information from its posterior region with affective-motivational inputs from its anterior region to shape the conscious experience of pain (Labrakakis, 2023; Lu et al., 2016; Mutschler et al., 2011). In chronic pain, synaptic plasticity within the insula, including long-term potentiation, heightens pain sensitivity and emotional distress, while its connectivity with the anterior cingulate cortex modulates these effects (Lee, Chenn, & Zhuo, 2022; Zhuo, 2016). Additionally, phenomena such as emotional allodynia—where emotional distress manifests as physical pain—underscore the insula’s role in linking affective and sensory dimensions of pain (Mutschler et al., 2011; Wang et al., 2021).
Addiction
The insular cortex is pivotal in addiction by integrating interoceptive signals with external cues, contributing to the development and maintenance of addictive behaviors. In cocaine addiction, for example, the anterior insula is implicated in the loss of control over drug intake; lesions in this area can restore control and prevent relapse (Rotge et al., 2017). Similarly, the rostral agranular insula influences nicotine self-administration and cue-induced reinstatement, while its role in the long-term memory of drug cravings further consolidates its significance in addiction (Toyoda, 2018). Altered insular activity—marked by hypoactivity during cognitive control and hyperactivity during cue reactivity—along with changes in synaptic plasticity, hypocretin transmission, and dopaminergic signaling (notably via D1 receptors), highlights its critical role in substance use disorders (Hollander et al., 2008; Kutlu et al., 2013). Functional and structural changes due to childhood adversity adapted from Szalavitz (2024) are shown below.

Autonomic Control
The insula is a key component of the central autonomic network, which includes the anterior cingulate cortex and amygdala, and plays a crucial role in regulating heart rate variability (HRV) (Chouchou et al., 2019; Szurhaj & De Jonckheere Orane Nicolas Reyns, 2019). The posterior insula typically mediates sympathetic responses (e.g., tachycardia), while the anterior insula is more involved in parasympathetic regulation (e.g., bradycardia). According to the neurovisceral integration model, the insula coordinates autonomic and cognitive functions, with high-frequency HRV—reflective of parasympathetic activity—correlating with its function, as evidenced by neuroimaging studies of the salience network (Colivicchi et al., 2004; Vargas et al., 2016). The central autonomic network adapted from Nikolin et al. (2017) is shown below.

Immunity
Emerging evidence indicates that the insular cortex encodes and retrieves immune responses. Neuronal ensembles in the insula, activated during specific inflammatory conditions in animal models, can, upon reactivation, recapitulate the original inflammatory state. This suggests that the insula not only encodes immune responses but may also initiate them, extending the concept of immunological memory to include neuronal representations (Koren et al., 2020).
Neurofeedback Interventions
Neurofeedback approaches targeting the insula have shown promise in modulating processes related to addiction and mindfulness. Real-time fMRI neurofeedback has been employed to decrease insular BOLD activity and reduce drug cravings in nicotine-addicted individuals (Rana et al., 2020). Additionally, neurofeedback-augmented mindfulness training in adolescents has demonstrated that modulating insular activity—by increasing anterior and decreasing posterior responses—can enhance interoceptive awareness and improve subjective well-being (Yu et al., 2022). These interventions suggest that targeting the insula may offer novel, non-pharmacological treatment avenues for various clinical conditions.
Key Takeaways
Integration of Processes: The insular cortex plays a central role in merging interoceptive signals with emotional and cognitive inputs, thereby forming our conscious experiences.
Distinct Subregional Functions: It is hierarchically organized into the posterior, middle, and anterior (dorsal and ventral) regions—each contributing uniquely to sensory reception, signal refinement, and processing of emotions and cognition.
Network Hub Role: The insula functions as a critical node within multiple brain networks (e.g., salience, sensorimotor, and frontoparietal), influencing attention allocation, decision-making, and cognitive control.
Clinical Relevance: Its multifaceted involvement in conditions such as chronic pain, addiction, and autonomic dysregulation underscores its potential as a target for therapeutic interventions, including mindfulness and neurofeedback.
Emerging Insights: Recent findings suggest the insula may also be involved in immune response encoding, broadening our understanding of its integrative functions across physiological and psychological domains.
Summary
The insular cortex, a complex brain region nestled within the lateral sulcus, is integral to transforming raw interoceptive data into conscious emotional experiences. Its hierarchical organization includes the posterior insula, which functions as a primary sensory reception area; the middle insula, which refines these signals; and the anterior insula, subdivided into dorsal regions involved in cognitive control and ventral regions responsible for emotional processing. This structured integration enables the insula to contribute to the perception of internal states, such as anxiety during a high-stress presentation.
Beyond its intrinsic processing capabilities, the insula is a critical node within several large-scale brain networks. It plays a central role in the salience network by mediating the switch between the default mode and executive networks, influencing attention allocation, decision-making, and emotional regulation. Its extensive connectivity with sensorimotor, limbic, and prefrontal regions underpins functions ranging from pain perception and empathy to interoception and cognitive control.
Clinically, the insula’s multifaceted role is evident in its involvement in various conditions, including chronic pain, addiction, and autonomic dysregulation. Interventions such as mindfulness practices and neurofeedback have been shown to modulate insular activity, enhancing interoceptive awareness and improving emotional regulation. Moreover, emerging research suggests that the insula may even participate in encoding immune responses, further underscoring its significance as an integrative hub linking physiological states to conscious experience.
Glossary
affective empathy: the capacity to share another’s emotional experiences.
addiction: a chronic disorder characterized by compulsive substance use despite negative consequences.
amygdala: a limbic structure involved in processing emotions and interoceptive signals.
anterior cingulate cortex: a brain region that collaborates with the insula in processing emotion, pain, and cognitive functions.
anterior insula: the frontal portion of the insular cortex, subdivided into dorsal and ventral regions, crucial for cognitive control, attention, and emotional processing.
attention: the cognitive process of selectively concentrating on relevant stimuli.
BOLD activity: blood oxygen level-dependent activity measured via functional imaging that reflects neural activation.
bradycardia: a decrease in heart rate associated with parasympathetic regulation.
central autonomic network: a circuit including the insula, anterior cingulate cortex, and amygdala that coordinates autonomic functions such as heart rate variability.
central executive network (CAN): a brain network engaged in high-level cognitive functions, modulated by the insula’s role in network switching.
chronic pain: pain persisting at least 3 months involving altered sensory and affective processing in the insula.
cognitive control: the ability to regulate thoughts and actions to achieve goal-directed behavior.
cognitive empathy: the ability to understand another’s mental state.
cognitive-affective processing: the integration of cognitive and emotional information.
connectivity: the pattern of neural connections between the insula and other brain regions.
cortical thickness: a measure of the thickness of the cerebral cortex, which can be influenced by behavioral interventions.
cocaine addiction: a substance use disorder related to cocaine, where insular dysfunction may contribute to loss of control over intake.
cue-induced reinstatement: the triggering of relapse by exposure to drug-related cues.
drug cravings: intense desires for drugs that arise from the integration of interoceptive and memory signals.
D1 receptors: a subtype of dopamine receptors involved in dopaminergic signaling affecting reward processes.
decision-making: the process of evaluating options and selecting actions based on integrated sensory, emotional, and cognitive inputs.
default mode network: a brain network active during rest and self-referential thought, modulated by the insula’s switching functions.
dorsal anterior insula: the upper subdivision of the anterior insula that supports attention and cognitive control.
dorsal prefrontal cortex: a frontal region involved in higher-order cognitive processes, functionally connected with the insula during attentional tasks.
dopaminergic signaling: neurotransmission mediated by dopamine, influencing reward processing and addictive behaviors.
emotional allodynia: a condition in which emotional distress produces a sensation of pain.
emotional regulation: the ability to modulate emotional responses, supported by insular processing.
empathy: the capacity to understand and share the feelings of others, involving both cognitive and affective components.
error-based learning: a process where feedback from mistakes informs future behavior adjustments.
exteroceptive inputs: sensory information from the external environment that is integrated with internal signals.
fronto-insular cortex: the portion of the insula that connects with frontal regions to facilitate network switching and cognitive control.
frontoparietal network: a brain network that supports high-level cognitive functions and interacts with the insula.
heart rate variability (HRV): the variation in time between heartbeats, indicative of autonomic regulation.
hypocretin transmission: neural signaling involving hypocretin that influences arousal and reward-related processes.
hypothalamus: a subcortical structure that maintains physiological homeostasis, interacting with the insula in processing interoceptive signals.
immunological memory: the capacity of neuronal circuits to encode and retrieve specific immune responses.
interoception: the process by which the brain monitors internal bodily states, primarily mediated by the insula.
interoceptive signals: physiological cues from within the body that are integrated to inform homeostatic and emotional states.
intracranial EEG: a technique for recording electrical activity directly from the brain, used to study insular function.
inflammatory conditions: states characterized by inflammation that can activate specific neuronal ensembles within the insula.
insular cortex: a region of the cerebral cortex located within the lateral sulcus that integrates sensory, emotional, and cognitive information.
insula: synonymous with the insular cortex; a concealed cortical region crucial for interoception, emotion, and cognitive integration.
lateral sulcus: a deep groove on the lateral aspect of the brain that houses the insula, complicating its imaging and accessibility.
limbic structures: brain regions involved in emotion and memory that interact with the insula.
long-term potentiation: a sustained increase in synaptic strength that underlies synaptic plasticity in processes such as pain and addiction.
middle insula: the transitional zone between the posterior and anterior insula that refines incoming sensory signals.
mindfulness: a practice promoting present-moment awareness that enhances interoception and emotional regulation through insular activity.
mindfulness-based interventions: therapeutic practices incorporating mindfulness techniques to modulate insular function and improve well-being.
neurofeedback interventions: techniques providing real-time feedback of brain activity.
neurovisceral integration model: a framework describing how the insula coordinates cognitive and autonomic functions.
neuronal ensembles: groups of neurons within the insula that collectively encode specific information, such as immune responses.
nicotine self-administration: a behavioral model of nicotine addiction influenced by insular function.
olfactory signals: smell-related sensory inputs processed by regions of the insula that contribute to the perception of bodily states.
posterior insula: the rear subdivision of the insula that receives raw interoceptive and other sensory data.
real-time fMRI neurofeedback: a technique using immediate functional imaging feedback to modulate insular activity for therapeutic purposes.
rostral agranular insula: a region implicated in addiction that influences behaviors such as nicotine self-administration and cue-induced relapse.
salience network: a large-scale brain network including the anterior insula that detects and prioritizes significant stimuli.
sensorimotor network: a network involved in processing sensory and motor information, with the posterior insula playing a central role.
somatosensory input: sensory information originating from the body that is processed by the insula during evaluative stages.
synaptic plasticity: the capacity of synapses to change their strength, underpinning learning, memory, and adaptive responses.
tachycardia: an increased heart rate typically mediated by sympathetic responses involving the posterior insula.
top-down processing: a cognitive process in which higher-order brain regions guide the interpretation of task-relevant information.
ventral anterior insula: the lower subdivision of the anterior insula that connects with limbic structures to support emotional processing.
References
Benarroch, E. (2019). Insular cortex. Neurology, 93, 932 - 938. https://doi.org/10.1212/WNL.0000000000008525 Bernhardt, B., & Singer, T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1-23 . https://doi.org/10.1146/annurev-neuro-062111-150536 Castelhano, J., Duarte, I., Ferreira, C., Durães, J., Madeira, H., & Castelo‐Branco, M. (2018). The role of the insula in intuitive expert bug detection in computer code: an fMRI study. Brain Imaging and Behavior, 13, 623 - 637. https://doi.org/10.1007/s11682-018-9885-1 Centanni, S., Janes, A., Haggerty, D., Atwood, B., & Hopf, F. (2021). Better living through understanding the insula: Why subregions can make all the difference. Neuropharmacology, 198. https://doi.org/10.1016/j.neuropharm.2021.108765 Chouchou, F., Mauguière, F., Vallayer, O., Catenoix, H., Isnard, J., Montavont, A., Jung, J., Pichot, V., Rheims, S., & Mazzola, L. (2019). How the insula speaks to the heart: Cardiac responses to insular stimulation in humans. Human Brain Mapping, 40, 2611 - 2622. https://doi.org/10.1002/hbm.24548 Cloutman, L., Binney, R., Drakesmith, M., Parker, G., & Ralph, M. (2012). The variation of function across the human insula mirrors its patterns of structural connectivity: Evidence from in vivo probabilistic tractography. NeuroImage, 59, 3514-3521. https://doi.org/10.1016/j.neuroimage.2011.11.016 Colivicchi, F., Bassi, A., Santini, M., & Caltagirone, C. (2004). Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke, 35, 2094-2098. https://doi.org/10.1161/01.STR.0000138452.81003.4c Evrard, H. (2019). The organization of the primate insular cortex. Frontiers in Neuroanatomy, 13. https://doi.org/10.3389/fnana.2019.00043 Ghaziri, J., Tucholka, A., Girard, G., Boucher, O., Houde, J., Descoteaux, M., Obaid, S., Gilbert, G., Rouleau, I., & Nguyen, D. (2018). Subcortical structural connectivity of insular subregions. Scientific Reports, 8. https://doi.org/10.1038/s41598-018-26995-0
Ghaziri, J., Tucholka, A., Girard, G., Houde, J., Boucher, O., Gilbert, G., Descoteaux, M., Lippé, S., Rainville, P., & Nguyen, D. (2017). The corticocortical structural connectivity of the human insula. Cerebral Cortex, 27, 1216–1228. https://doi.org/10.1093/cercor/bhv308
Gu, X., Hof, P., Friston, K., & Fan, J. (2013). Anterior insular cortex and emotional awareness. Journal of Comparative Neurology, 521. https://doi.org/10.1002/cne.23368
Haruki, Y., & Ogawa, K. (2021). Role of anatomical insular subdivisions in interoception: Interoceptive attention and accuracy have dissociable substrates. European Journal of Neuroscience, 53, 2669 - 2680. https://doi.org/10.1111/ejn.15157
Hollander, J., Lu, Q., Cameron, M., Kamenecka, T., & Kenny, P. (2008). Insular hypocretin transmission regulates nicotine reward. Proceedings of the National Academy of Sciences, 105, 19480 - 19485. https://doi.org/10.1073/pnas.0808023105
Jumah, F. (2018). Role of the insular cortex in emotional awareness. In M. Turgut, C. Yurttaş, & R. Tubbs (Eds.), Island of Reil (insula) in the human brain. Springer. https://doi.org/10.1007/978-3-319-75468-0_18
Koren, T., Krot, M., Boshnak, N., Amer, M., Ben-Shaanan, T., Azulay‐Debby, H., Hajjo, H., Avishai, E., Schiller, M., Haykin, H., Korin, B., Cohen-Farfara, D., Hakim, F., Rosenblum, K., & Rolls, A. (2020). Remembering immunity: Neuronal ensembles in the insular cortex encode and retrieve specific immune responses. bioRxiv. https://doi.org/10.1101/2020.12.03.409813
Kurth, F., Zilles, K., Fox, P., Laird, A., & Eickhoff, S. (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function, 214, 519-534. https://doi.org/10.1007/s00429-010-0255-z
Kutlu, M., Burke, D., Slade, S., Hall, B., Rose, J., & Levin, E. (2013). Role of insular cortex D1 and D2 dopamine receptors in nicotine self-administration in rats. Behavioural Brain Research, 256, 273-278. https://doi.org/10.1016/j.bbr.2013.08.005
Labrakakis, C. (2023). The role of the insular cortex in pain. International Journal of Molecular Sciences, 24. https://doi.org/10.3390/ijms24065736
Lee, J., Chen, Q., & Zhuo, M. (2022). Synaptic plasticity in the pain-related cingulate and insular cortex. Biomedicines, 10. https://doi.org/10.3390/biomedicines10112745
Li, R., Zhang, S., Yin, S., Ren, W., He, R., & Li, J. (2018). The fronto‐insular cortex causally mediates the default‐mode and central‐executive networks to contribute to individual cognitive performance in healthy elderly. Human Brain Mapping, 39, 4302 - 4311. https://doi.org/10.1002/hbm.24247
Llorens, A., Bellier, L., Blenkmann, A., Ivanović, J., Larsson, P., Lin, J., Endestad, T., Solbakk, A., & Knight, R. (2022). Decision and response monitoring during working memory are sequentially represented in the human insula. iScience, 26. https://doi.org/10.1016/j.isci.2023.107653
Lu, C., Yang, T., Zhao, H., Zhang, M., Meng, F., Fu, H., Xie, Y., & Xu, H. (2016). Insular cortex is critical for the perception, modulation, and chronification of pain. Neuroscience Bulletin, 32, 191-201. https://doi.org/10.1007/s12264-016-0016-y
Menon, V., Gallardo, G., Pinsk, M., Nguyen, V., Li, J., Cai, W., & Wassermann, D. (2020). Microstructural organization of human insula is linked to its macrofunctional circuitry and predicts cognitive control. eLife, 9. https://doi.org/10.7554/eLife.53470
Menon, V., & Uddin, L. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214, 655-667. https://doi.org/10.1007/s00429-010-0262-0
Mutschler, I., Wankerl, J., Seifritz, E., & Ball, T. (2011). The role of the human insular cortex in pain processing. European Psychiatry, 26, 1001 - 1001. https://doi.org/10.1016/S0924-9338(11)72706-7
Naqvi, N., & Bechara, A. (2009). The hidden island of addiction: The insula. Trends in Neurosciences, 32, 56-67. https://doi.org/10.1016/j.tins.2008.09.009
Nelson, S., Dosenbach, N., Cohen, A., Wheeler, M., Schlaggar, B., & Petersen, S. (2010). Role of the anterior insula in task-level control and focal attention. Brain Structure & Function, 214, 669 - 680. https://doi.org/10.1007/s00429-010-0260-2
Nikolin, S., Boonstra, T. W., Loo, C. K., & Martin, D. (2017). Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PloS one, 12(8), e0181833. https://doi.org/10.1371/journal.pone.0181833
Nieuwenhuys, R. (2012). The insular cortex: a review. Progress in Brain Research, 195, 123-63 . https://doi.org/10.1016/B978-0-444-53860-4.00007-6
Nomi, J., Farrant, K., Damaraju, E., Rachakonda, S., Calhoun, V., & Uddin, L. (2016). Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Human Brain Mapping, 37. https://doi.org/10.1002/hbm.23135
Nomi, J., Schettini, E., Broce, I., Dick, A., & Uddin, L. (2018). Structural connections of functionally defined human insular subdivisions. Cerebral Cortex, 28, 3445–3456. https://doi.org/10.1093/cercor/bhx211
Rana, M., Ruiz, S., Corzo, A., Muehleck, A., Eck, S., Salinas, C., Zamorano, F., Silva, C., Rea, M., Batra, A., Birbaumer, N., & Sitaram, R. (2020). Use of real-time functional magnetic resonance imaging-based neurofeedback to downregulate insular cortex in nicotine-addicted smokers. Journal of Visualized Experiments: JoVE, 160. https://doi.org/10.3791/59441
Rotge, J., Cocker, P., Daniel, M., Belin-Rauscent, A., Everitt, B., & Belin, D. (2017). Bidirectional regulation over the development and expression of loss of control over cocaine intake by the anterior insula. Psychopharmacology, 234, 1623 - 1631. https://doi.org/10.1007/s00213-017-4593-x
Schimmelpfennig, J., Topczewski, J., Zajkowski, W., & Jankowiak-Siuda, K. (2023). The role of the salience network in cognitive and affective deficits. Frontiers in Human Neuroscience, 17, 1133367. https://doi.org/10.3389/fnhum.2023.1133367
Shelley, B., & Trimble, M. (2004). The insular Lobe of Reil–Its anatamico-functional, behavioural and neuropsychiatric attributes in humans–A review. The World Journal of Biological Psychiatry, 5, 176 - 200. https://doi.org/10.1080/15622970410029933
Singer, T., Critchley, H., & Preuschoff, K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13, 334-340. https://doi.org/10.1016/j.tics.2009.05.001 Sridharan, D., Levitin, D., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105, 12569 - 12574. https://doi.org/10.1073/pnas.0800005105 Szalavitz, M. (2024). New treatments address addiction alongside trauma. Scientific American, 331(3), 44. https://doi.org/10.1038/scientificamerican102024-6xB36RNXDLNTqrJNtnh0wL Szurhaj, W., & De Jonckheere Orane Nicolas Reyns, P. (2019). O-02 Study of the cortical control of the cardiac autonomic nervous system using intracerebral electrical stimulations. Clinical Neurophysiology, 130. https://doi.org/10.1016/j.clinph.2019.04.318 Toyoda, H. (2018). Nicotine facilitates synaptic depression in layer V pyramidal neurons of the mouse insular cortex. Neuroscience Letters, 672, 78-83. https://doi.org/10.1016/j.neulet.2018.02.046 Vargas, R., Sörös, P., Shoemaker, J., & Hachinski, V. (2016). Human cerebral circuitry related to cardiac control: A neuroimaging meta‐analysis. Annals of Neurology, 79. https://doi.org/10.1002/ana.24642 Wang, N., Zhang, Y., Wang, J., & Luo, F. (2021). Current understanding of the involvement of the insular cortex in neuropathic pain: A narrative review. International Journal of Molecular Sciences, 22. https://doi.org/10.3390/ijms22052648 Wang, X., Wu, Q., Egan, L., Gu, X., Liu, P., Gu, H., Yang, Y., Luo, J., Wu, Y., Gao, Z., & Fan, J. (2018). Anterior insular cortex plays a critical role in interoceptive attention. eLife, 8. https://doi.org/10.7554/eLife.42265 Wang, X., Wu, Q., Egan, L., Gu, X., Liu, P., Gu, H., Yang, Y., Luo, J., Wu, Y., Gao, Z., & Fan, J. (2019). Anterior insular cortex plays a critical role in interoceptive attention. eLife, 8. https://doi.org/10.7554/ELIFE.42265 Wheeler, M., Arnkoff, D., & Glass, C. (2017). The neuroscience of mindfulness: How mindfulness alters the brain and facilitates emotion regulation. Mindfulness, 8, 1471-1487. https://doi.org/10.1007/S12671-017-0742-X Young, K., Velden, A., Craske, M., Pallesen, K., Fjorback, L., Roepstorff, A., & Parsons, C. (2018). The impact of mindfulness-based interventions on brain activity: A systematic review of functional magnetic resonance imaging studies. Neuroscience & Biobehavioral Reviews, 84, 424-433. https://doi.org/10.1016/j.neubiorev.2017.08.003 Yu, X., Cohen, Z., Tsuchiyagaito, A., Cochran, G., Aupperle, R., Stewart, J., Singh, M., Misaki, M., Bodurka, J., Paulus, M., & Kirlic, N. (2022). Neurofeedback-augmented mindfulness training elicits distinct responses in the subregions of the insular cortex in healthy adolescents. Brain Sciences, 12. https://doi.org/10.3390/brainsci12030363 Zhang, Y., Zhou, W., Wang, S., Zhou, Q., Wang, H., Zhang, B., Huang, J., Hong, B., & Wang, X. (2019). The roles of subdivisions of human insula in emotion perception and auditory processing. Cerebral Cortex, 29, 517–528. https://doi.org/10.1093/cercor/bhx334
Zhuo, M. (2016). Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience, 338, 220-229. https://doi.org/10.1016/j.neuroscience.2016.08.014
Zsadanyi, S., Kurth, F., & Luders, E. (2021). The effects of mindfulness and meditation on the cingulate cortex in the healthy human brain: A review. Mindfulness, 12, 2371 - 2387. https://doi.org/10.1007/s12671-021-01712-7
Support Our Friends





Comments