The Basal Ganglia Are More Than a Motor Circuit
- BioSource Faculty
- Dec 15, 2024
- 14 min read

The basal ganglia, a collection of subcortical nuclei, have long been recognized for their pivotal role in motor control. However, recent advances in neuroscience have expanded our understanding of these structures, revealing their critical involvement in a broad range of cognitive, affective, and motivational processes.
These nuclei, situated deep within the brain, serve as essential hubs that integrate and modulate information across various neural circuits. This essay examines the multifaceted functions of the basal ganglia, exploring their influence on cognition, emotion, and motivation, and underscoring their significance in both normal and pathological states.
Basal Ganglia Anatomy
The basal ganglia are a group of subcortical nuclei deeply embedded within the cerebral hemispheres, playing a critical role in regulating motor control, cognitive functions, and emotional processing. The primary components of the basal ganglia include the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra. Each of these structures is located in specific brain regions and participates in distinct neural circuits that connect with various cortical and subcortical areas.
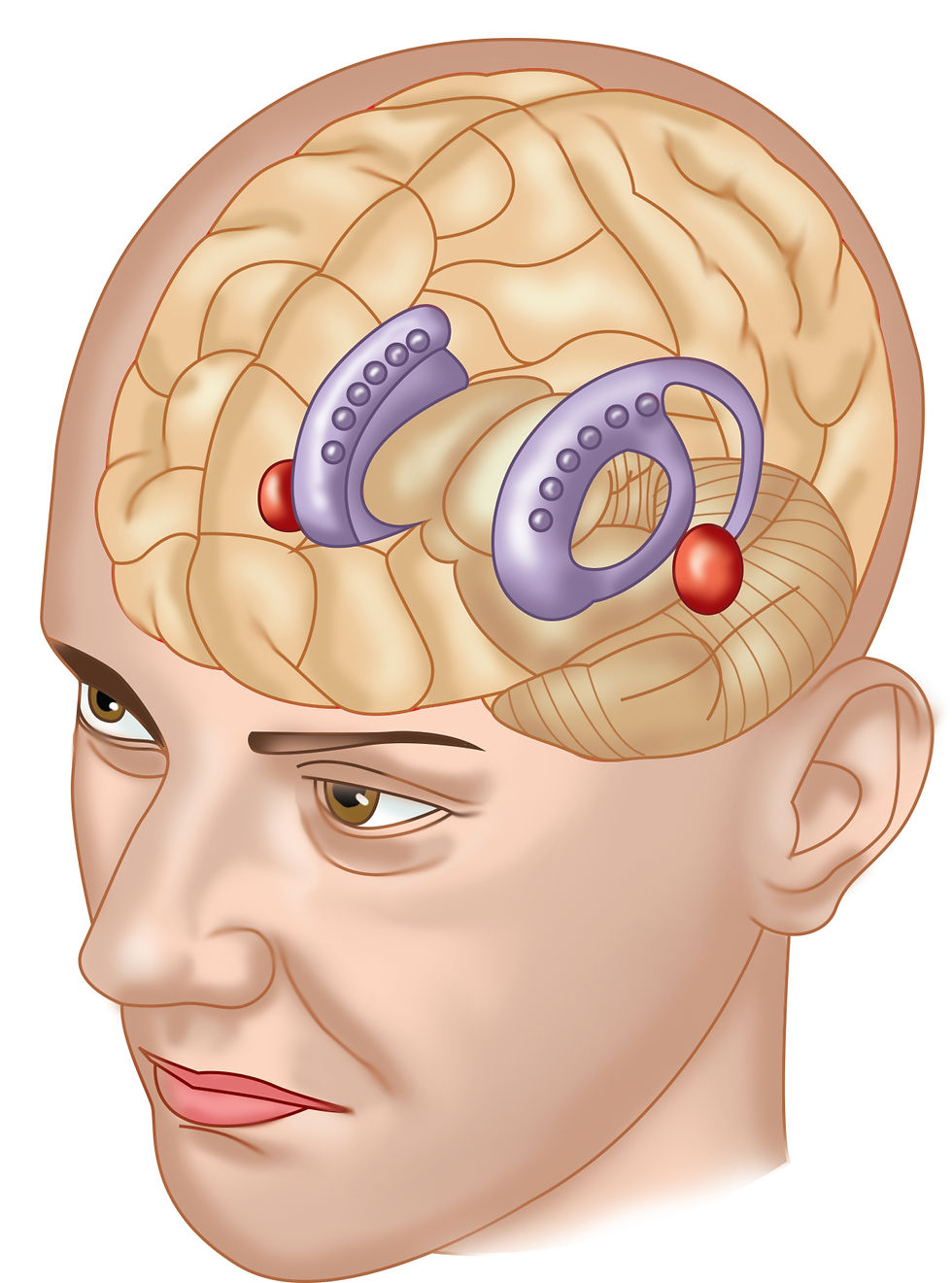
The caudate nucleus and putamen, collectively known as the striatum, form the largest component of the basal ganglia. The striatum is located in the forebrain, with the caudate nucleus curving around the lateral ventricles, and the putamen lying lateral to the globus pallidus.
The striatum serves as the primary input region of the basal ganglia, receiving extensive projections from the cerebral cortex, particularly from the frontal, parietal, and temporal lobes, as well as from the thalamus (Lindroos et al., 2018). The striatum is crucial for the integration of cortical inputs and for modulating motor, cognitive, and emotional outputs through its connections with other basal ganglia components (Lanciego, Luquin, & Obeso, 2012).
The globus pallidus, situated medial to the putamen, is divided into two segments: the external segment (GPe) and the internal segment (GPi). The GPi is a major output nucleus of the basal ganglia, projecting to the thalamus and relaying information back to the cerebral cortex. This connection forms part of the direct and indirect pathways of the basal ganglia circuits, which are essential for controlling the initiation and suppression of movements (Albin, Young, & Penney, 1989). The GPe, on the other hand, is primarily involved in the indirect pathway, where it regulates the activity of the subthalamic nucleus and influences the inhibitory control exerted by the GPi. Basal ganglia graphic redrawn from Sejnowski et al. (2013).

The subthalamic nucleus (STN) is a small, lens-shaped structure located below the thalamus and above the substantia nigra. The STN plays a key role in the indirect pathway of the basal ganglia, where it receives inhibitory input from the GPe and sends excitatory projections to the GPi and substantia nigra pars reticulata (SNr). The STN's activity is crucial for the modulation of motor control, particularly in suppressing unwanted movements and regulating motor precision (Temel et al., 2005).
The substantia nigra, located in the midbrain, is divided into two parts: the pars compacta (SNc) and the pars reticulata (SNr). The SNc contains dopaminergic neurons that project to the striatum, forming the nigrostriatal pathway. This pathway is vital for regulating movement, as dopamine released by the SNc modulates the activity of the striatum, influencing both the direct and indirect pathways of the basal ganglia circuits (Bolam et al., 2000). The SNr, similar to the GPi, acts as an output nucleus, sending inhibitory projections to the thalamus and brainstem, thus playing a role in motor control and the regulation of eye movements.
The basal ganglia participate in several key neural circuits, called cortico-basal ganglia-thalamo-cortical loops. These loops include the motor circuit, which is involved in the planning and execution of voluntary movements; the associative circuit, which is related to cognitive functions such as working memory and decision-making; and the limbic circuit, which is associated with emotional processing and motivational behavior (Alexander, DeLong, & Strick, 1986). Each of these circuits involves distinct areas of the cortex, basal ganglia, and thalamus, allowing for the integration and modulation of diverse brain functions.
In summary, the basal ganglia comprise several interconnected nuclei, each with a specific location and role within the brain. The caudate nucleus and putamen (striatum), globus pallidus, subthalamic nucleus, and substantia nigra form complex circuits that regulate motor, cognitive, and emotional processes. These circuits are essential for the proper functioning of various neural systems and are implicated in a wide range of neurological and psychiatric disorders.
The Basal Ganglia Role in Psychological Disorders
This section examines the role of the basal ganglia in psychological disorders, focusing on conditions such as Parkinson's disease, Huntington's disease, obsessive-compulsive disorder (OCD), and schizophrenia.
Parkinson’s Disease
Parkinson’s disease (PD) is one of the most well-known disorders associated with basal ganglia dysfunction. This neurodegenerative disorder primarily affects the motor system, leading to symptoms such as tremors, rigidity, bradykinesia (slowness of movement), and postural instability.
The underlying pathology of Parkinson’s involves the progressive degeneration of dopaminergic neurons in the substantia nigra, a key component of the basal ganglia (Kalia & Lang, 2015; Mehler-Wex et al., 2006). The loss of dopamine, which is critical for the normal functioning of the basal ganglia circuits, disrupts the balance between the direct and indirect pathways that modulate motor activity, resulting in the characteristic motor symptoms of the disease.
PD is a neurodegenerative disorder primarily affecting the basal ganglia, leading to motor and non-motor symptoms.
Beyond motor symptoms, Parkinson's disease also involves significant non-motor symptoms, including cognitive impairment, mood disorders, and personality changes. Cognitive deficits in Parkinson’s, particularly in executive function, memory, and attention, have been linked to dysfunction in the basal ganglia-thalamocortical circuits, which are responsible for integrating cognitive processes (Jellinger, 2012). Mood disturbances, such as depression and anxiety, are also common in Parkinson’s and are thought to result from disruptions in the basal ganglia’s connections with the limbic system, highlighting the role of these structures in regulating mood and affect.
Huntington’s Disease
Huntington’s disease (HD) is another neurodegenerative disorder that is closely associated with basal ganglia dysfunction. Unlike Parkinson’s, which primarily involves the loss of dopaminergic neurons, Huntington’s disease is characterized by the progressive degeneration of neurons in the striatum, particularly the caudate nucleus and putamen (Ross & Tabrizi, 2011; Stoko et al., 2010). This degeneration leads to a range of symptoms, including motor abnormalities, cognitive decline, and psychiatric disturbances.
The motor symptoms of Huntington’s disease, such as chorea (involuntary, jerky movements) and dystonia (muscle contractions leading to abnormal postures), are directly related to the loss of neurons in the striatum, which disrupts the normal functioning of the basal ganglia circuits. However, the cognitive and psychiatric symptoms of Huntington’s are equally debilitating and often precede the onset of motor symptoms. Cognitive impairments in Huntington’s include deficits in executive function, memory, and visuospatial abilities, while psychiatric symptoms can range from depression and irritability to more severe conditions such as psychosis (Paulsen, 2011). The widespread impact of basal ganglia degeneration in Huntington’s disease underscores the importance of these structures in maintaining normal cognitive and emotional functioning.
Obsessive-Compulsive Disorder
Obsessive-compulsive disorder (OCD) is a psychiatric condition characterized by intrusive thoughts (obsessions) and repetitive behaviors (compulsions) that the individual feels driven to perform. The basal ganglia, particularly the striatum, have been implicated in the pathophysiology of OCD, with dysfunction in the cortico-striato-thalamo-cortical (CSTC) circuits playing a central role (Hollander et al., 1993; Pauls et al., 2014). These circuits are involved in the regulation of habitual behaviors and the inhibition of inappropriate actions, both of which are disrupted in OCD.
Neuroimaging studies have shown abnormalities in the structure and function of the basal ganglia in individuals with OCD, particularly in the caudate nucleus and putamen. These abnormalities are thought to contribute to the difficulty that individuals with OCD have in controlling compulsive behaviors and in shifting their focus from obsessive thoughts (Saxena & Rauch, 2000). Moreover, the basal ganglia’s involvement in reward processing and habit formation suggests that the repetitive behaviors seen in OCD may be related to maladaptive learning processes, further implicating these structures in the disorder.
Schizophrenia
Schizophrenia is a complex psychiatric disorder characterized by a range of symptoms, including hallucinations, delusions, cognitive impairment, and emotional dysregulation. The basal ganglia have been implicated in the pathophysiology of schizophrenia, particularly through their role in dopamine regulation. Dopaminergic dysfunction, particularly in the striatum, has long been associated with the positive symptoms of schizophrenia, such as hallucinations and delusions (Halje et al., 2019; Howes & Kapur, 2009).
The basal ganglia, through their connections with the prefrontal cortex and other cortical regions, are also involved in cognitive processes often impaired in schizophrenia, such as working memory, attention, and executive function. Dysfunction in the basal ganglia-thalamocortical circuits is thought to contribute to these cognitive deficits, as well as to the negative symptoms of schizophrenia, such as anhedonia and social withdrawal (Simpson et al., 2010). Additionally, the involvement of the basal ganglia in emotional regulation suggests that abnormalities in these structures may also contribute to the affective disturbances observed in schizophrenia.
Summary
The basal ganglia play a critical role in a wide range of psychological disorders, extending far beyond their traditional association with motor control. From neurodegenerative diseases like Parkinson’s and Huntington’s to psychiatric conditions such as OCD and schizophrenia, the dysfunction of basal ganglia circuits has profound implications for cognitive, emotional, and behavioral health. Understanding the role of the basal ganglia in these disorders not only enhances our knowledge of their underlying mechanisms but also informs the development of more targeted and effective treatments. As research continues to uncover the complex functions of the basal ganglia, their significance in health and disease will become increasingly evident.

Cognitive Functions
The basal ganglia contribute significantly to cognitive processes, particularly those related to executive functions, such as decision-making, working memory, and attention. One of the key pathways linking the basal ganglia to cognitive function is the cortico-basal ganglia-thalamo-cortical loop, which involves the prefrontal cortex, the striatum (a major component of the basal ganglia), the globus pallidus, and the thalamus (Middleton & Strick, 2000). This loop facilitates the integration and processing of cognitive information, enabling the basal ganglia to influence higher-order cognitive functions.
Studies have demonstrated that the basal ganglia, particularly the striatum, play a pivotal role in the selection and initiation of cognitive actions, a process often referred to as cognitive control (Robbins, 2007). Specific regions within the basal ganglia, like the caudate nucleus, are crucial for goal-directed actions and the evaluation of action outcomes, distinguishing them from regions like the putamen, which are more involved in habit learning (Grahn et al., 2008). The basal ganglia plays a role in action-selection processes needed for the expression of declarative and procedural memories (Da Cunha et al., 2012). The caudate nucleus contributes to behavior through the excitation of correct action schemas and the selection of appropriate sub-goals based on an evaluation of action-outcomes (Grahn et al., 2008). The basal ganglia help filter out irrelevant information, allowing for the focused execution of tasks by prioritizing certain cognitive actions over others. This selection process is critical for maintaining attention and goal-directed behavior, particularly in complex environments where distractions are prevalent.
Moreover, the basal ganglia are involved in working memory processes. Working memory, the ability to temporarily hold and manipulate information, is essential for many cognitive tasks, such as problem-solving and decision-making. Research suggests that the basal ganglia contribute to the updating and maintenance of working memory by modulating the activity of the prefrontal cortex (Frank et al., 2001). Dysfunction in this system, as seen in conditions like Parkinson's disease, often leads to deficits in working memory and other executive functions, highlighting the importance of the basal ganglia in cognitive processing.
Affective Functions
In addition to their cognitive roles, the basal ganglia are deeply involved in affective processing, particularly in regulating emotions. The limbic circuit, which includes the ventral striatum (notably the nucleus accumbens), the amygdala, the hippocampus, and the prefrontal cortex, forms a pathway through which the basal ganglia influence emotional responses (Arsalidou et al., 2013; Haber & Knutson, 2010; Martino et al., 2008). This circuit is critical for processing emotional stimuli, mediating reward-related behavior, and regulating mood.
The basal ganglia's role in affective functions is most prominently observed in reward processing and reinforcement learning. The ventral striatum, through its connections with the dopaminergic system, particularly the substantia nigra and the ventral tegmental area, plays a crucial role in encoding reward prediction errors—discrepancies between expected and actual outcomes (Schultz, 2007). This process is essential for learning from experience and adapting behavior based on emotional feedback.
Moreover, the basal ganglia are implicated in the modulation of mood and the expression of emotions. Dysfunctions in the basal ganglia, particularly in the striatal pathways, have been associated with affective disorders such as depression and anxiety (Price & Drevets, 2010). For example, decreased activity in the ventral striatum has been linked to anhedonia, a core symptom of depression characterized by a reduced ability to experience pleasure. This evidence underscores the basal ganglia's critical role in affective processing and emotional regulation.
Motivational Functions
The basal ganglia are also integral to regulating motivation, particularly in goal-directed behavior and the pursuit of rewards. The nucleus accumbens, a part of the ventral striatum, is central to the motivational functions of the basal ganglia. It acts as a hub where cognitive, affective, and motivational signals converge, influencing the drive to initiate and sustain goal-directed activities (Berridge & Robinson, 2003).
Motivation is closely tied to reinforcement learning, where the basal ganglia play a critical role in evaluating the potential rewards or punishments associated with different actions. Through the dopaminergic system, particularly the dopamine release in the nucleus accumbens, the basal ganglia help signal the motivational value of a stimulus, thereby influencing decision-making and behavior selection (Pierce et al., 2020; Wise, 2004). This process is fundamental for adaptive behavior, enabling individuals to prioritize actions that maximize rewards and minimize negative outcomes.
Additionally, the basal ganglia regulate effort-related decision-making, where the perceived cost of an action (in terms of effort) is weighed against the potential reward (Salamone & Correa, 2012). Dysfunction in this system, such as reduced dopamine transmission in the basal ganglia, can lead to motivational deficits, as observed in disorders like Parkinson's disease and depression, where individuals often exhibit reduced initiative and a lack of motivation to engage in activities.
Conclusion
The basal ganglia are far more than mere regulators of movement; they are integral to the complex interplay of cognitive, emotional, and motivational processes that underpin human behavior. By participating in diverse neural circuits, these structures influence everything from decision-making and memory to emotional regulation and goal-directed behavior. The dysfunction of basal ganglia circuits has profound implications, contributing to the pathophysiology of numerous neurological and psychiatric disorders. As our understanding of the basal ganglia continues to evolve, so too will our ability to develop targeted interventions that address the wide-ranging effects of their dysfunction, ultimately improving outcomes for individuals affected by these conditions.
Google Illuminate Discussion
Please click on the podcast icon to listen to a lively Google Illuminate discussion of this post.
Glossary
anhedonia: the inability to experience pleasure from activities typically found enjoyable, often associated with depression.
associative circuit: a neural circuit within the basal ganglia involved in cognitive functions such as working memory and decision-making.
basal ganglia: a group of subcortical nuclei located deep within the brain, involved in motor control, as well as cognitive, affective, and motivational functions.
caudate nucleus: a component of the basal ganglia, part of the striatum, involved in motor processes, as well as cognitive functions such as goal-directed behavior.
chorea: involuntary, rapid, and irregular movements, typically associated with Huntington's disease.
cortico-basal ganglia-thalamo-cortical loop: neural circuits that connect the cortex, basal ganglia, thalamus, and back to the cortex, playing a crucial role in regulating motor, cognitive, and emotional functions.
cortico-striato-thalamo-cortical (CSTC) circuits: neural pathways connecting the cortex, striatum, thalamus, and back to the cortex, implicated in the regulation of motor, cognitive, and emotional processes.
cognitive control: the ability to regulate thought processes and actions, especially in terms of decision-making, working memory, and attention.
dopaminergic neurons: neurons that produce and release dopamine, a neurotransmitter involved in reward, motivation, and motor control.
dystonia: a movement disorder characterized by sustained muscle contractions leading to abnormal postures or repetitive movements.
effort-related decision-making: the process of evaluating the cost of an action in terms of effort versus the potential reward, influenced by basal ganglia function.
executive function: a set of cognitive processes, including working memory, flexible thinking, and self-control, that are essential for managing and regulating behavior.
globus pallidus: a component of the basal ganglia involved in the regulation of voluntary movement, divided into the external (GPe) and internal (GPi) segments.
GPe (external globus pallidus): a component of the basal ganglia involved in the indirect pathway, regulating motor control by influencing the subthalamic nucleus.
GPi (internal globus pallidus): a component of the basal ganglia that serves as a major output nucleus, playing a key role in the direct pathway by relaying information to the thalamus to modulate movement.
Huntington's disease (HD): a genetic neurodegenerative disorder characterized by motor dysfunction, cognitive decline, and psychiatric symptoms, caused by the degeneration of neurons in the striatum.
limbic circuit: a neural circuit involving the basal ganglia, amygdala, hippocampus, and prefrontal cortex, essential for emotional processing and reward-related behavior.
motivational functions: the processes that drive goal-directed behavior, influenced by the basal ganglia through the evaluation of rewards and the initiation of actions.
nucleus accumbens: a part of the ventral striatum involved in the processing of rewards, motivation, and reinforcement learning.
Parkinson's disease (PD): a neurodegenerative disorder characterized by motor symptoms such as tremors and rigidity, resulting from the loss of dopaminergic neurons in the substantia nigra.
pars compacta (SNc): a part of the substantia nigra in the midbrain, containing dopaminergic neurons that project to the striatum, essential for movement regulation.
pars reticulata (SNr): a part of the substantia nigra involved in the output of the basal ganglia, sending inhibitory signals to the thalamus and brainstem, and involved in motor control.
procedural memory: a type of long-term memory involved in the acquisition of skills and habits, often associated with the functioning of the basal ganglia.
putamen: a component of the striatum within the basal ganglia, involved in motor control and certain types of learning.
reinforcement learning: a type of learning in which behavior is shaped by rewards or punishments, with the basal ganglia playing a key role in evaluating outcomes.
striatum: the largest component of the basal ganglia, composed of the caudate nucleus and putamen, and involved in motor, cognitive, and emotional processes.
substantia nigra: a part of the basal ganglia located in the midbrain, involved in motor control through its dopaminergic connections with the striatum.
subthalamic nucleus (STN): a component of the basal ganglia involved in the regulation of motor control, particularly in suppressing unwanted movements.
ventral striatum: A portion of the striatum, including the nucleus accumbens, involved in reward processing and motivation.
References
Arsalidou, M., Duerden, E., & Taylor, M. (2013). The centre of the brain: Topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Human Brain Mapping, 34. https://doi.org/10.1002/hbm.22124.
Berridge, K. C., & Robinson, T. E. (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. https://doi.org/10.1016/S0166-2236(03)00233-9
Da Cunha, C., Gomez-A, A., & Blaha, C. D. (2012). The role of the basal ganglia in motivated behavior. Reviews in the Neurosciences, 23(5-6), 747–767. https://doi.org/10.1515/revneuro-2012-0063
Frank, M. J., Loughry, B., & O'Reilly, R. C. (2001). Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive, Affective & Behavioral Neuroscience, 1(2), 137–160. https://doi.org/10.3758/cabn.1.2.137
Grahn, J., Parkinson, J., & Owen, A. (2008). The cognitive functions of the caudate nucleus. Progress in Neurobiology, 86, 141-155. https://doi.org/10.1016/j.pneurobio.2008.09.004
Haber, S. N., & Knutson, B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 4–26. https://doi.org/10.1038/npp.2009.129
Halje, P., Brys, I., Mariman, J. J., da Cunha, C., Fuentes, R., & Petersson, P. (2019). Oscillations in cortico-basal ganglia circuits: implications for Parkinson's disease and other neurologic and psychiatric conditions. Journal of neurophysiology, 122(1), 203–231. https://doi.org/10.1152/jn.00590.2018
Hollander, E., Cohen, L., Richards, M., Mullen, L., Decaria, C., & Stern, Y. (1993). A pilot study of the neuropsychology of obsessive-compulsive disorder and Parkinson's disease: Basal ganglia disorders. The Journal of Neuropsychiatry and Clinical Neurosciences, 5(1), 104-107. https://doi.org/10.1176/JNP.5.1.104
Lindroos, R., Dorst, M., Du, K., Filipovic, M., Keller, D., Ketzef, M., Kozlov, A., Kumar, A., Lindahl, M., Nair, A., Pérez-Fernández, J., Grillner, S., Silberberg, G., & Kotaleski, J. (2018). Basal ganglia neuromodulation over multiple temporal and structural scales—Simulations of direct pathway MSNs investigate the fast onset of dopaminergic effects and predict the role of Kv4.2. Frontiers in Neural Circuits, 12. https://doi.org/10.3389/fncir.2018.00003
Martino, A., Scheres, A., Margulies, D., Kelly, A., Uddin, L., Shehzad, Z., Biswal, B., Walters, J., Castellanos, F., & Milham, M. (2008). Functional connectivity of human striatum: A resting state FMRI study. Cerebral Cortex, 18(12), 2735-2747. https://doi.org/10.1093/cercor/bhn041
Mehler-Wex, C., Riederer, P., & Gerlach, M. (2006). Dopaminergic dysbalance in distinct basal ganglia neurocircuits: Implications for the pathophysiology of parkinson’s disease, schizophrenia and attention deficit hyperactivity disorder. Neurotoxicity Research, 10, 167-179. https://doi.org/10.1007/BF03033354
Middleton, F. A., & Strick, P. L. (2000). Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research. Brain Research Reviews, 31(2-3), 236–250. https://doi.org/10.1016/s0165-0173(99)00040-5
Pierce, J., & Péron, J. (2020). The basal ganglia and the cerebellum in human emotion. Social Cognitive and Affective Neuroscience, 15, 599 - 613. https://doi.org/10.1093/scan/nsaa076
Price, J. L., & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 192–216. https://doi.org/10.1038/npp.2009.104
Robbins T. W. (2007). Shifting and stopping: Fronto-striatal substrates, neurochemical modulation and clinical implications. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 362(1481), 917–932. https://doi.org/10.1098/rstb.2007.2097
Salamone, J. D., & Correa, M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76(3), 470–485. https://doi.org/10.1016/j.neuron.2012.10.021
Schultz W. (2007). Multiple dopamine functions at different time courses. Annual Review of Neuroscience, 30, 259–288. https://doi.org/10.1146/annurev.neuro.28.061604.135722
Stocco, A., Lebiere, C., & Anderson, J. (2010). Conditional routing of information to the cortex: a model of the basal ganglia's role in cognitive coordination. Psychological Review, 117(2), 541-574. https://doi.org/10.1037/a0019077
Wise R. A. (2004). Dopamine, learning and motivation. Nature Reviews. Neuroscience, 5(6), 483–494. https://doi.org/10.1038/nrn1406
Support Our Friends









Comments